SERVICES
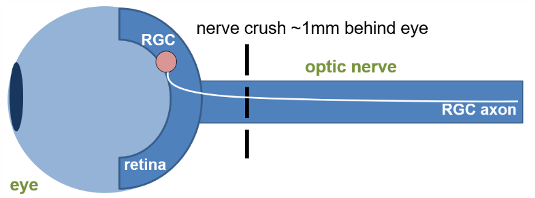
Figure 1. Schematic representation of the method of induction.
Glaucoma is a collection of diseases that lead to irreversible vision loss as a result of retinal ganglion cell (RGC) death and their subsequent inability to transmit information to the visual cortex.1 The optic nerve crush (ONC) surgical model involves a precise and consistent injury to the optic nerve, thereby leading to the RGC death that is consistent with glaucoma and other neurodegenerative disorders of the retina. Using reliable in-life and post-life readouts gives us insight into disease pathology and can shed light on potential therapeutics.
| Animal Species | Mouse |
| Method of Induction | Crush injury to optic nerve using forceps |
| Follow up Period | Acutely following injury through 4-6 weeks |
| Route of intervention | Intravitreal, intracameral, topical, systemic |
| Readouts |
|
Central nervous system trauma and neurodegenerative disorders can trigger a cascade of cellular and molecular events culminating in neuronal apoptosis. The Optic Nerve Crush model provides an effective tool for analyzing the pathogenic mechanisms associated with neuronal injury signaling in vivo. Optic nerve crush has been used as a model neuronal injury, including glaucoma, traumatic optic neuropathies, neurodegeneration and CNS injury. Crush injury to the optic nerve severs the retinal ganglion cell (RGC) axons leading to the gradual death of RGC neurons in the retina. The model provides an opportunity to study neuronal outcomes following injury, including survival, apoptosis, regeneration and associated biomarkers. Applications include traumatic optic neuropathy, glaucoma and neurodegenerative disease.
Optic nerve crush serves as a useful model for traumatic optic neuropathy and mimics glaucomatous injury, similarly inducing RGC cell death and degeneration. Glaucomatous injury is a pathohistological feature of glaucoma in the optic nerve.
Molecular Readouts Illustration Model Induction
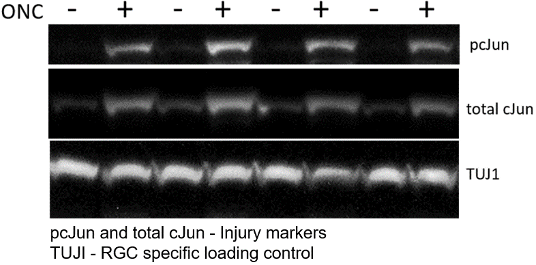
Multidimensional observations strengthen the interpretation: in addition to in-life measurements (i.e., ERG), Immunostaining monitors therapeutic effect, immunoassays track biomarkers, and qRT-PCR provides information on retinal gene expression. Markers tracked in this model include:
| Proten/Gene | Significance |
|---|---|
| pcJun | neuronal injury |
| TUJI | RGC marker |
| Atf3 | regeneration-associated genes |
| Sprr1a | regeneration-associated genes |
| Ddit3 (Chop) | pro-apoptotic transcription factor |
| Gfap | Reactive astrocyte marker |
Treating and Reversing Glaucoma
The GD3 Ocular Center of Excellence is proud to offer efficacy models in which physiological readouts coupled with cellular and biochemical measurements provide a comprehensive snapshot of your treatment's therapeutic potential. The optic nerve crush model can test agents treating glaucoma, traumatic optic neuropathies, neurodegeneration, and CNS injury and inflammation. If your organization is working to treat any of these debilitating diseases, we encourage you to examine our capabilities:
- Glaucoma
- In vivo Mouse Model for Glaucoma
- Traumatic Optic Neuropathies
- CNS Injury
- Inflammation
- Neurodegenerative Diseases
Readouts
Optic Nerve Crush Use in Your Research Program
Optic nerve crush allows for the evaluation of drug intervention following neuronal injury at the cellular and biochemical levels. Immunostaining can monitor therapeutic effects, and immunoassays can be developed to track biomarkers following treatment.